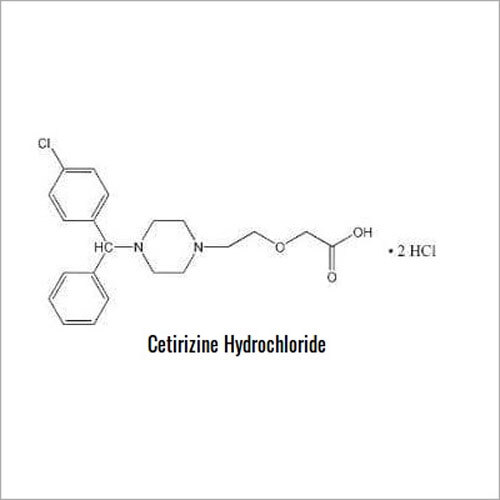
Cetirizine Dihyrochloride IP / EP / USP is an excellent pharmaceutical-grade compound, known for its valuable antihistamine properties. With a kingly purity assay from 99.0% to 101.0%, it offers instant results upon use. Its appearance is a white to off-white powder, freely soluble in water. Featuring a melting point of approximately 218C and a robust shelf life of 36 months, this product conforms to global standards including chloride content and heavy metals. Secure your purchase through instant checkout and order today from India's trusted exporter, manufacturer, and supplier.
Cetirizine Dihyrochloride IP / EP / USP: Versatile Applications
Cetirizine Dihyrochloride IP / EP / USP finds valuable application in pharmaceutical plants, especially in the production of anti-allergy medications. Its features include high assay accuracy and reliable solubility, making it compatible with advanced machine processing and formulation systems. In addition to pharma, it is also used in biotechnology and chemical research, highlighting its versatility and excellent quality for a range of applications.
Packaging, Certifications, and Sample Supply Details
Packing expenditure is minimized thanks to secure fibre drums with double polyethylene bags, conforming to international safeguards. The product is supplied as per sale price agreements and customer requirements. Certified under IP/EP/USP specifications, each batch maintains kingly quality standards. Samples are available for evaluation before large-scale use, making your purchasing process efficient and transparent.
Cetirizine Dihyrochloride IP / EP / USP: Versatile Applications
Cetirizine Dihyrochloride IP / EP / USP finds valuable application in pharmaceutical plants, especially in the production of anti-allergy medications. Its features include high assay accuracy and reliable solubility, making it compatible with advanced machine processing and formulation systems. In addition to pharma, it is also used in biotechnology and chemical research, highlighting its versatility and excellent quality for a range of applications.
Packaging, Certifications, and Sample Supply Details
Packing expenditure is minimized thanks to secure fibre drums with double polyethylene bags, conforming to international safeguards. The product is supplied as per sale price agreements and customer requirements. Certified under IP/EP/USP specifications, each batch maintains kingly quality standards. Samples are available for evaluation before large-scale use, making your purchasing process efficient and transparent.
FAQ's of Cetirizine Dihyrochloride IP / EP / USP:
Q: How should Cetirizine Dihyrochloride IP / EP / USP be stored for optimal shelf life?
A: Cetirizine Dihyrochloride IP / EP / USP must be stored in a cool, dry place, protected from light, to maintain its 36-month shelf life and preserve its valuable properties.Q: What is the benefit of using Cetirizine Dihyrochloride IP / EP / USP in pharmaceutical production?
A: This compound ensures excellent purity and consistency, conforming to IP/EP/USP standards, allowing manufacturers to produce high-quality antihistamine formulations efficiently.Q: Where is Cetirizine Dihyrochloride IP / EP / USP exported, and who are the suppliers?
A: Cetirizine Dihyrochloride IP / EP / USP is exported from India by certified manufacturers, suppliers, and importers, offering global reach with reliable quality.Q: When is sample availability offered for this product?
A: Samples of Cetirizine Dihyrochloride IP / EP / USP are available before bulk orders, allowing evaluation and expenditure planning for your business needs.Q: What process is followed for packaging and maintaining product safety?
A: The compound is packed in fibre drums with double polyethylene bags to prevent contamination and meet international safety guidelines before supply.Q: How is the sale price determined for Cetirizine Dihyrochloride IP / EP / USP?
A: Sale price depends on quantity, packaging requirements, and certifications, ensuring competitive expenditure for every purchase.Related Products
© Copyright 2023 Hema Pharma. All Rights Reserved Copyright