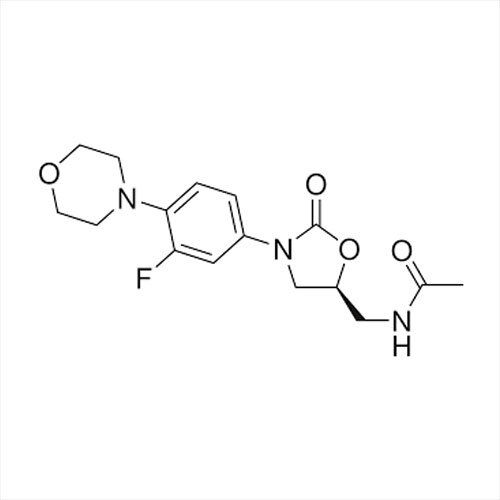
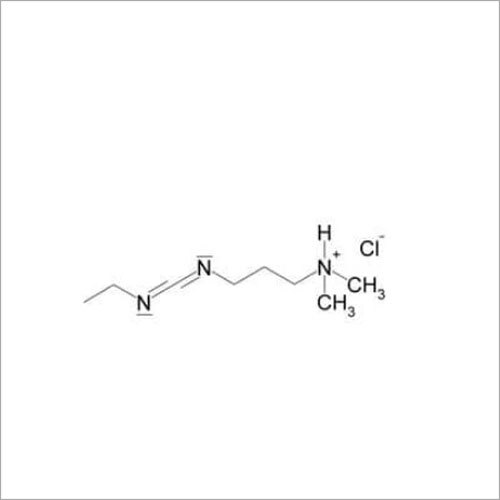
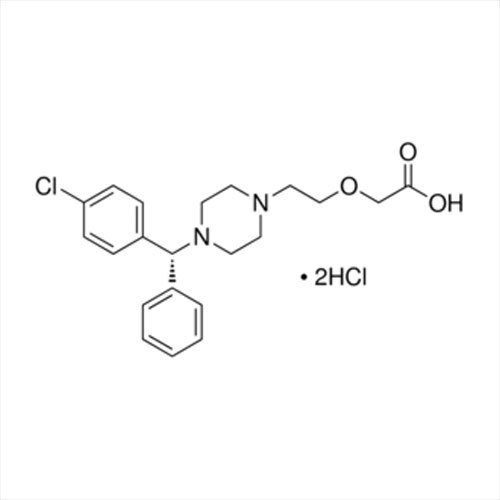
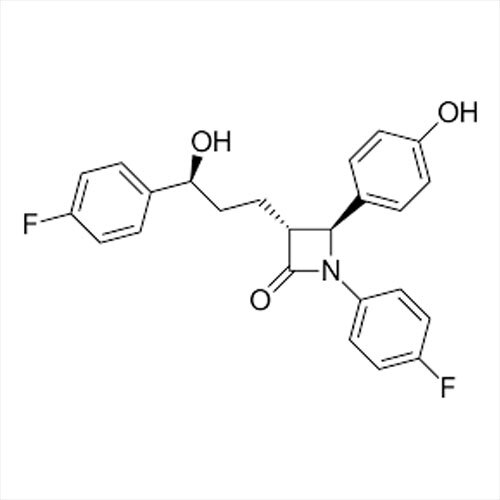
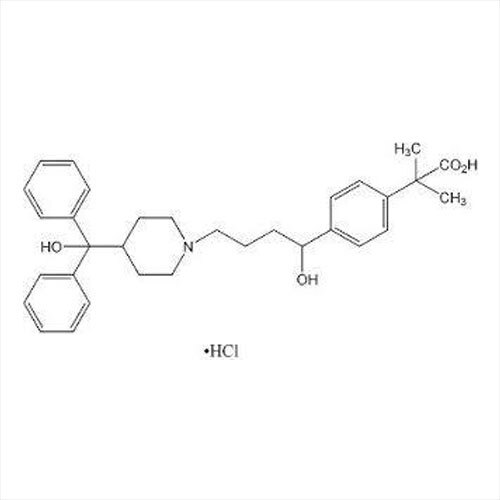
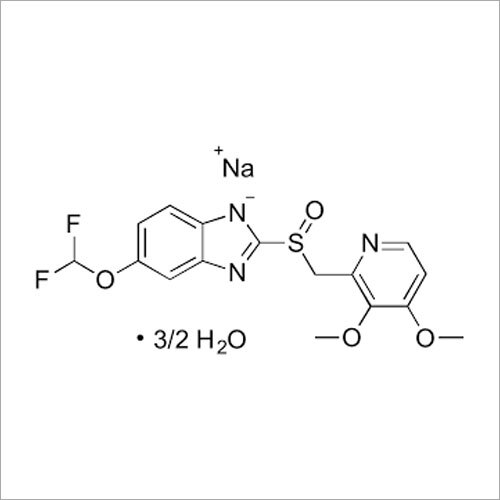
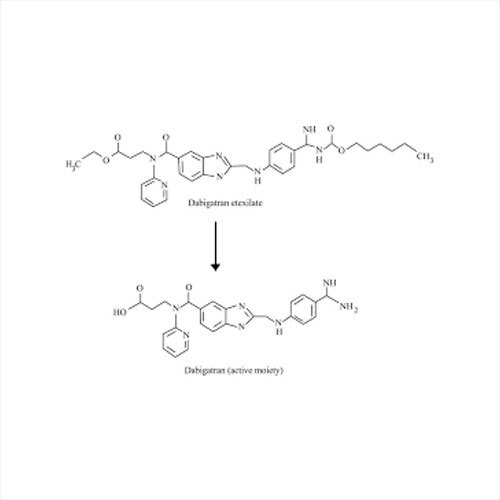
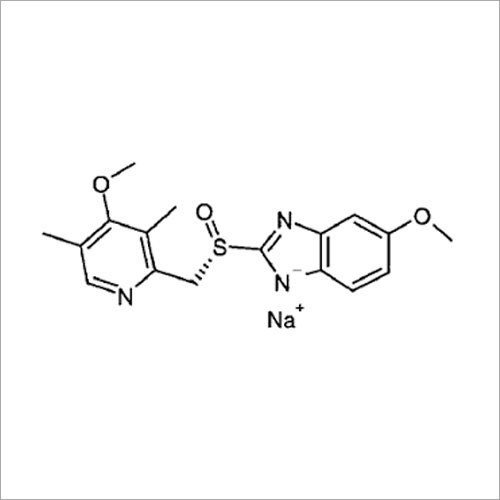
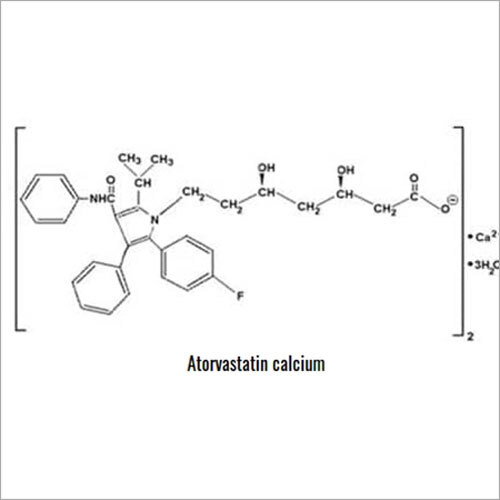
Grab the affordable Secubitril IP/BP/USP, a top choice among pharmaceutical intermediates with a prodigious purity of 99% min. This terrific compound, formulated as a white to off-white powder, promises towering performance in high-demand pharma applications. With a molecular formula of C22H25NO4 and a remarkable melting point of 115-120C, Secubitril assures reliability. Soluble in DMSO and methanol, it is available in customizable HDPE drum packaging. Shelf life extends two years, maintaining quality under cool, dry, well-ventilated conditions. Exported, imported, and supplied by leading Indian manufacturers.
Secubitril IP/BP/USP: Applications and User Profile
Secubitril IP/BP/USP is widely utilized by pharmaceutical manufacturers, exporters, and suppliers due to its outstanding purity. Used for synthesizing critical medical intermediates, it serves the thriving healthcare industry and research laboratories alike. With its versatile powder form, Secubitril is perfect for application on diverse pharmaceutical production surfaces, ensuring consistent results for top-tier medicinal compound development.
Secubitril IP/BP/USP: Delivery Times, Certifications, and Supply
Secubitril IP/BP/USP comes with a prompt delivery timeline, ensuring customers receive their orders without undue delay. Upon quotation and exchange, formal certifications validate its IP/BP/USP grade, demonstrating compliance with international standards. With prolific supply ability, bulk shipments are managed seamlessly, tailored packaging provided, and reliable logistics implemented so clients can trust in every delivery to maintain consistently high standards.
Secubitril IP/BP/USP: Applications and User Profile
Secubitril IP/BP/USP is widely utilized by pharmaceutical manufacturers, exporters, and suppliers due to its outstanding purity. Used for synthesizing critical medical intermediates, it serves the thriving healthcare industry and research laboratories alike. With its versatile powder form, Secubitril is perfect for application on diverse pharmaceutical production surfaces, ensuring consistent results for top-tier medicinal compound development.
Secubitril IP/BP/USP: Delivery Times, Certifications, and Supply
Secubitril IP/BP/USP comes with a prompt delivery timeline, ensuring customers receive their orders without undue delay. Upon quotation and exchange, formal certifications validate its IP/BP/USP grade, demonstrating compliance with international standards. With prolific supply ability, bulk shipments are managed seamlessly, tailored packaging provided, and reliable logistics implemented so clients can trust in every delivery to maintain consistently high standards.
FAQ's of Secubitril IP/BP/USP:
Q: How should Secubitril IP/BP/USP be properly stored to maintain its efficacy?
A: Secubitril IP/BP/USP should be stored in a cool, dry, well-ventilated area to preserve its quality and extend its shelf life of up to two years.Q: What is the recommended process for dissolving Secubitril IP/BP/USP?
A: Secubitril IP/BP/USP is soluble in DMSO and methanol. Simply add the appropriate solvent to the powder and mix thoroughly for complete dissolution.Q: When will delivery of Secubitril IP/BP/USP typically occur after an order is placed?
A: Delivery times for Secubitril IP/BP/USP are prompt, and will be confirmed with the customer during the quotation and order exchange process.Q: Where can Secubitril IP/BP/USP be applied during pharmaceutical production?
A: Secubitril IP/BP/USP is designed for diverse surfaces in pharmaceutical manufacturing environments, especially within intermediate synthesis processes.Q: What benefits does Secubitril IP/BP/USP offer to manufacturers and suppliers?
A: Secubitril IP/BP/USP offers prodigious purity, reliable performance, and flexible packaging options, making it a top choice for manufacturers and suppliers in pharmaceutical applications.Related Products
© Copyright 2023 Hema Pharma. All Rights Reserved Copyright