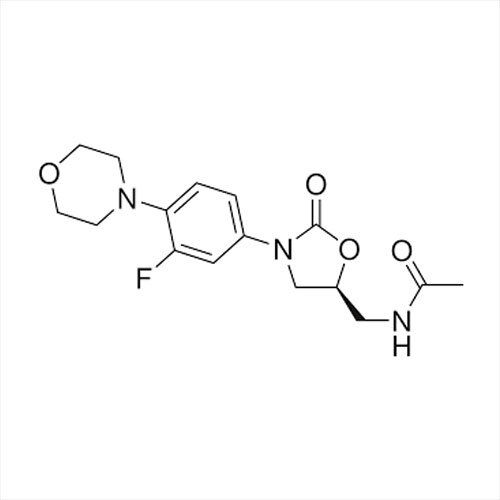
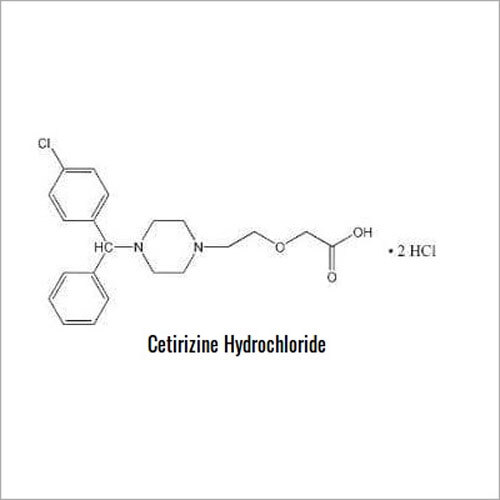
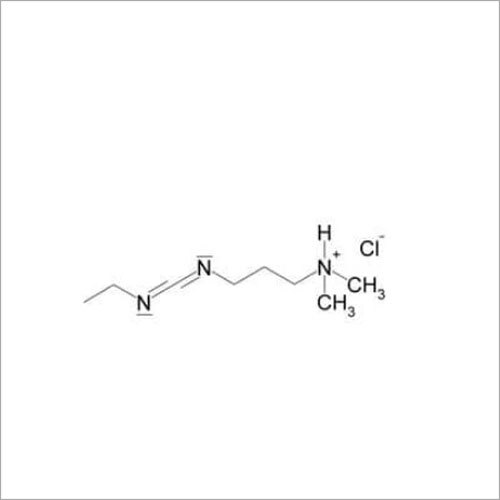
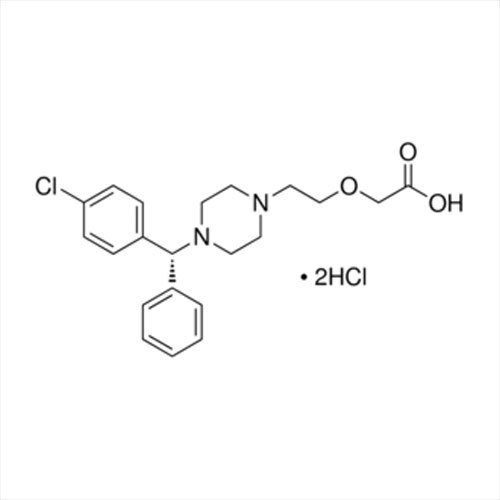
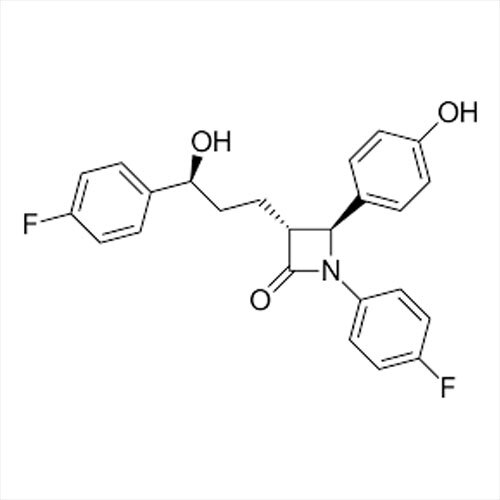
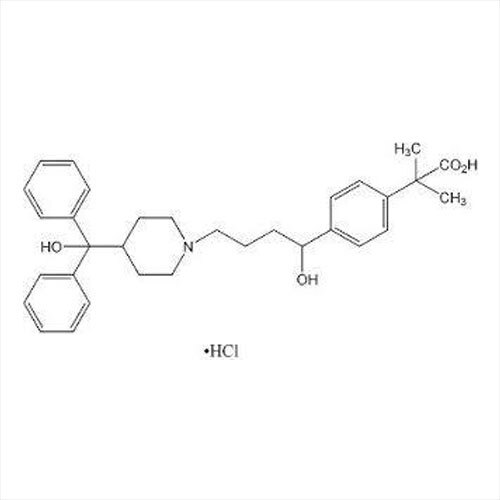
Introducing the latest Tamsulosin HCL-IP/BP/USP grade, a potent pharmaceutical intermediate offering unparalleled purity of 99% min (HPLC Assay). This phenomenal compound, presented as a white crystalline powder, is identified by IR and HPLC, ensuring instant savings in quality control and optimal assurance. With a shelf life of five years and exceptional solubility in water and methanol, Tamsulosin HCL is used for the effective treatment of benign prostatic hyperplasia. Explore the benefits of a product compliant with global standards, meticulously packed in 25 kg HDPE drums for secure transport and storage. Manufactured, supplied, imported, and exported from India, it guarantees reliability for pharmaceutical formulations.
Extra Features & Specific Usage
Tamsulosin HCL-IP/BP/USP grade boasts a high melting point (108-110C), minimal loss on drying, and stringent heavy metal controls. Its phenomenal efficacy makes it especially valuable as a pharmaceutical intermediate in medications targeting benign prostatic hyperplasia. General and specific uses center around reliable formulation in tablet or capsule form, optimizing patient outcome with its unparalleled purity. These extra features position Tamsulosin HCL as a trusted backbone in modern pharmaceutical manufacturing.
Main Export Markets, Certifications & Delivery
Tamsulosin HCL is exported globally, with established main markets across North America, Europe, and Asia, reflecting its widespread trust and certification to IP/BP/USP standards. Our refined packaging-25 kg HDPE drums-ensures safety and integrity during goods transport. Packing & dispatch are streamlined for prompt delivery, typically within the agreed lead times to maintain client satisfaction and meet regulatory demands. The certification guarantees compliance, making it a preferred choice for international pharmaceutical partners.
Extra Features & Specific Usage
Tamsulosin HCL-IP/BP/USP grade boasts a high melting point (108-110C), minimal loss on drying, and stringent heavy metal controls. Its phenomenal efficacy makes it especially valuable as a pharmaceutical intermediate in medications targeting benign prostatic hyperplasia. General and specific uses center around reliable formulation in tablet or capsule form, optimizing patient outcome with its unparalleled purity. These extra features position Tamsulosin HCL as a trusted backbone in modern pharmaceutical manufacturing.
Main Export Markets, Certifications & Delivery
Tamsulosin HCL is exported globally, with established main markets across North America, Europe, and Asia, reflecting its widespread trust and certification to IP/BP/USP standards. Our refined packaging-25 kg HDPE drums-ensures safety and integrity during goods transport. Packing & dispatch are streamlined for prompt delivery, typically within the agreed lead times to maintain client satisfaction and meet regulatory demands. The certification guarantees compliance, making it a preferred choice for international pharmaceutical partners.
FAQ's of Tamsulosin HCL- IP/BP/USP:
Q: How should Tamsulosin HCL be stored to maintain its potency?
A: Tamsulosin HCL should be stored at room temperature, protected from light and moisture, in its original packaging (25 kg HDPE drum) to preserve its potency and stability throughout its five-year shelf life.Q: What are the primary uses of Tamsulosin HCL in the pharmaceutical industry?
A: Tamsulosin HCL is primarily used as a pharmaceutical intermediate for the treatment of benign prostatic hyperplasia. It's incorporated into medications, typically in tablet or capsule form, to help improve urinary flow and reduce symptoms associated with this condition.Q: What makes Tamsulosin HCL stand out in terms of quality and purity?
A: Tamsulosin HCL stands out due to its unparalleled purity, with an assay of 99% minimum (HPLC), low heavy metal content, and stringent loss on drying criteria, ensuring a high-quality, reliable product for pharmaceutical manufacturers.Q: Where are the main export markets for Tamsulosin HCL?
A: Tamsulosin HCL is majorly exported to North America, Europe, and Asia, where pharmaceutical standards demand products certified to IP/BP/USP grades and superior purity.Q: What is the packing and dispatch process for Tamsulosin HCL shipments?
A: Tamsulosin HCL is packed in secure 25 kg HDPE drums. After careful inspection and certification checks, goods are dispatched using reliable transport partners, ensuring swift and safe delivery to international clients.Q: How quickly can Tamsulosin HCL be delivered after order confirmation?
A: Delivery timelines typically depend on destination and customs regulations; however, packing and dispatch are efficiently managed to provide timely shipment and meet client schedules.Related Products
© Copyright 2023 Hema Pharma. All Rights Reserved Copyright